Our laboratory, Teppei Yamada Laboratory, or Inorganic Chemistry Laboratory, is based on both synthetic chemistry and property chemistry, and aiming to realize our own original research concepts and functionalities.
We have proposed an original thermoelectric conversion system by incorporating the essence of various physical property such as intermolecular interactions, proton-coupled electron transfer reactions, host-guest chemistry, and phase transition phenomena into thermo-electrohcemical cells, which are thermoelectric conversion materials utilizing the temperature dependence of the redox reaction equilibrium.
We are also working on our own unique ideas in a wide variety of other synthetic and physical property fields, such as specially designed plastic crystals, flexible functional molecules using conjugation between the σ orbital of silicon and the π orbital of carbon, and metal-organic frameworks with ion-recognition spaces.
Many of these studies may not have been heard of on TV or in the news before. We are proud of that. We are taking on the challenge of conducting research that goes even further beyond the “cutting-edge chemical technology you’ve heard about somewhere else.”
Thermo-electrochemical cells with molecular technology
*We recently published a review article in Angewandte Chemie.
A thermo-electrochemical cell, whichi is also called as thermocell or thermogalvanic cell, is a minor thermoelectric conversion device that uses a redox reaction of a solution to generate electricity from heat. We have created and developed thermocells by introducing various molecular technologies such as host-guest reactions, proton-conjugated electron transfer reactions, and phase transitions in polymers. We are reaching a stage where practical applications are fully foreseeable.

For more details, please consult the review paper.
Plastic Ionic Crystals
Generally, a substance has three phases: solid, liquid, and gas. However, by the sophisticate control of intermolecular interactions, we can create intermediate phases between solid and liquid. A plastic crystal phase is one of such solid-liquid intermediate phase, in which molecules and ions are periodically arranged in rotational motion at a site. Many plastic crystals have a texture similar to candle wax. Plastic crystals made of ionic pairs (plastic ionic crystals) have recently attracted attention as solid (-like) electrolytes and thermal media.
We have created a number of new materials exhibiting a plastic ionic crystal phase by using synthetic chemistry. Some of them have been found to rotate on one axis instead of spherical, and one of the ions is just flapping. We have discovered these new phases and are investigating their application development as ionic conductors and heat media.

Electrochemical Phase Transition Phenomena
Water changes its kinetic state drastically at 0 °C and 1 atm. Similarly, molecular aggregates rapidly change their aggregation state at a certain temperature and pressure, as in the coil-grobule transition of polymers. We are searching for new phases using electrical stimuli.
Ion-selective metal-organic frameworks
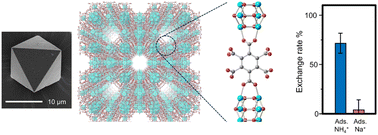
Metal-organic frameworks (MOFs) or coordination polymers are jungle gym-like structures formed by coordination bonding of metal ions and organic ligands. MOFs have nano-sized space in their crystals the size of which is comparable to those of molecules, and can be recognized as crystalline supramolecular hosts. A variety of MOFs have been synthesized by selecting metal ions and ligands, and their adsorption properties for various gas molecules have been reported depending on the gap size and chemical properties.
Our group has created a new MOF that stably absorbs ions in water. We have also succeeded in selectively extracting ammonium ions from solutions containing a variety of ions. We expect to find many other interesting properties by using the controlled pores of MOFs.
Flexible Functional Molecules Based on the Conjugation of the σ-Orbitals of Silicon and the π-Orbitals of Carbon
The π-conjugated system of carbon exhibits various physical properties such as luminescence, conductivity, and coordination ability. In addition, the structure is fixed by sp2 hybridization. On the other hand, disilane compounds consisting of two silicon atoms can construct conjugated systems via σ-bonds. σ-bonds have a degree of freedom of rotation, which enables to create interesting functional materials with flexible conjugated systems.
We have pioneered the synthesis of these conjugated disilane compounds and obtained a variety of new disilane compounds. Interestingly, these compounds can significantly change their color (i.e., electronic structure) and luminescence properties in response to mechanical stimuli such as grinding, solvents, and temperature.
We are exploring the diverse physical properties of flexible conjugated systems.
Our lab just started in 2020. We are working on new themes and have already found some interesting phenomena. We are looking for graduate students who can enjoy the moment when a new research theme is launched from scratch together with us.